Column Category
Attention to details: Why March Scopes are filled with Argon gas
Posted 09/01/2023
Many riflescope manufacturers use Nitrogen gas as they are cheap. Argon gas accounts for only 0.93% of the atmosphere and is considered as one of the noble gases. Argon gas is much more expensive than Nitrogen gas, but all March Scopes are Argon gas filled for the reasons mentioned below.
1. Argon is superior to Nitrogen as it does not absorb water molecules. It provides long-term protection against fogging and corrosion inside optical products.
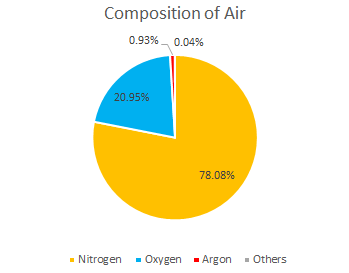
2. Argon gas is an “inert gas” that does not react with other substances and is non-toxic and non-flammable. Its inertness (the property of not reacting with other substances) is higher than that of Nitrogen. Nitrogen is inert at room temperature, but may react at high temperatures, while Argon, a group 18 gas, is quite stable and does not react even under high temperature conditions. Not only is Argon a noble gas, but also for this reason, it is much more expensive than Nitrogen.
3. The size of the Argon atom is much larger than the Nitrogen molecule, allowing it to remain inside the optics longer than the Nitrogen molecule. March riflescopes are filled with Argon gas after the air is slowly evacuated over time to create a vacuum.
DEON (manufacturer of March Scopes) is committed to making the best products, not only in what you see, but also in the details that you cannot see. Thank you for your continued support!
Written by: Mari Morita

